valence electrons of gold|valence electrons chart : Bacolod Step-2 is very important. In this step, the electrons of gold have to be arranged. We know that gold atoms have a total of seventy-nine electrons. The full electron configuration of gold is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1. Here the valence electron configuration of gold is . Tingnan ang higit pa at stake翻譯:有風險;處於危急關頭。了解更多。
valence electrons of gold,Step-2 is very important. In this step, the electrons of gold have to be arranged. We know that gold atoms have a total of seventy-nine electrons. The full electron configuration of gold is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1. Here the valence electron configuration of gold is . Tingnan ang higit paThe elements in the periodic table are arranged according to their atomic number. Since rhodium is the 79th element of . Tingnan ang higit pa
The third step is to diagnose the valence shell. The last shell after the electron configuration is called the valence shell. The total number of electrons in a valence shell is . Tingnan ang higit pa Valences of the Elements Chemistry Table. You may assume that the .
Mar 23, 2023 Gold Valence Electrons Dot Diagram. You can study the electron valence of Gold with better insight by using the dot diagram of elements. The diagram will .
To find the number of valence electrons for Gold (Au) we need to look at its electron configuration. This is necessary because Au is a transition metal (d b.
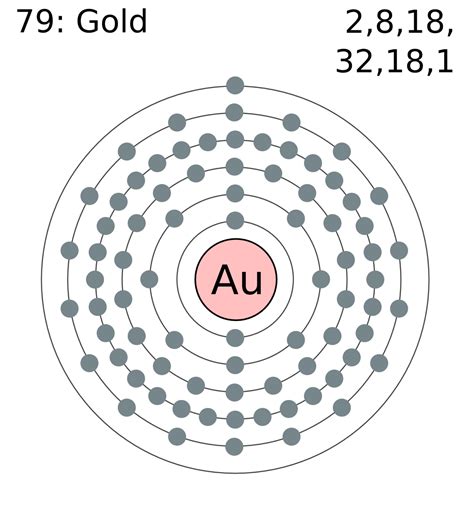
The atomic number is the number of electrons in that element. The atomic number of gold is 79. That is, the number of electrons in gold is seventy-nine. Therefore, a gold atom will have two electrons in the first shell, .The periodic table and Lewis diagrams. Determine valence electrons using the periodic table. Google Classroom. Learn how to determine the number of valence electrons for .
Overview. Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy . For a main-group element, the .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) .
About. Transcript. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, . Copper, aluminium, silver, and gold are examples of good conductors. A nonmetallic element has low electrical conductivity; it acts as an insulator. Such an element is found toward the right of the periodic table, .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..Gold is a chemical element of the periodic table with chemical symbol Au and atomic number 79 with an atomic weight of 196.967 u and is classed as a transition metal. . Valence electrons : 1: Valency electrons : 1,3: Bohr model: Electron shell for Gold, created by Injosoft AB Au. Figure: Shell diagram of Gold (Au) atom. Orbital Diagram. 1s .valence electrons of gold valence electrons chart Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
Valency: The valency of an atom is defined as the number of electrons gained or lost from the valence shell by an atom to attain noble gas electronic configuration. Gold belongs to the d-block element of group 11 and 6th period. The atomic number of Gold(Au)=79; The electronic configuration of Gold is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 .valence electrons chart 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. Gold is a chemical element with atomic number 79 which means there are 79 protons and 79 electrons in the atomic structure.The chemical symbol for Gold is Au. Electron Configuration and Oxidation States of Gold. Electron configuration of Gold is [Xe] 4f14 5d10 6s1. Possible oxidation states are +1,3. Electron Configuration. The .

To write the orbital diagram of gold, you have to write the orbital notation of gold. Which has been discussed in detail above. Gold orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital.

To write the orbital diagram of gold, you have to write the orbital notation of gold. Which has been discussed in detail above. Gold orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electrons will first enter the 1s orbital.Gold. 79. 196.967. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Valence describes how easily an atom or radical can combine with other chemical species. This is determined based on the number of electrons that would be added, lost, or shared if it reacts with .
valence electrons of gold The number of valence electrons in an atom may have the same or different numerical value as its oxidation state. For example, a lithium atom has 1 valence electron and has an oxidation state of +1. In . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd . Bulk properties of metals. Metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity (so they will give up electrons easily to form cations). Their physical properties include a lustrous (shiny) appearance, and they are malleable and ductile. . These are homework exercises to accompany Chapter 4 of the Furman University's LibreText for CHE 101 - Chemistry and Global Awareness. 4: Valence Electrons and Bonding. Valence electrons are outer shell electrons with an atom and can participate in the formation of chemical bonds. In single covalent bonds, typically .
$\begingroup$ Gold (Au) is a d-block element and has 1 valence electron (sometimes 3 or even 11 depending on how “valence electron is defined.) However Gold most often has chemical reactions with 1 or 3 valence electrons. Reactions with three valence electrons would be marked as gold(III.)
With one valence electron, gold exhibits a range of chemical behaviors that make it a highly versatile element in various applications. The electron configuration of gold, 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s1, reveals that the valence electron is located in the 6s orbital. This lone electron in the outermost energy .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
valence electrons of gold|valence electrons chart
PH0 · valence electrons chart
PH1 · valence electrons calculator
PH2 · number of valence electrons list
PH3 · list of valence electrons for each element
PH4 · how to find valence electrons
PH5 · ground state electron configuration gold
PH6 · electron configuration of gold
PH7 · bohr dot diagram of gold
PH8 · Iba pa